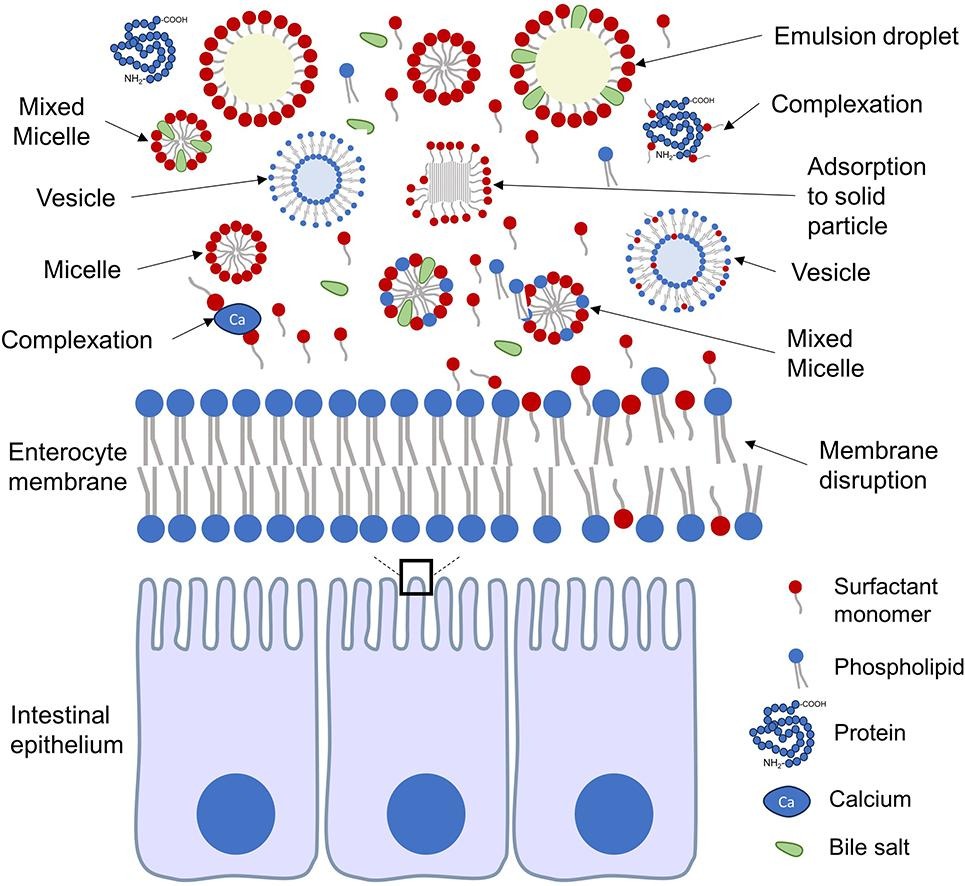
In our ADDR paper, “Safety of Surfactant Excipients in Oral Drug Formulations” by Sam Maher and colleagues, we reviewed scientific literature that explore the safety issues around extra materials placed in tablets and capsules, apart from the drug itself.
The extra substances are called “excipients” and they are added to oral drug formulations for a number of reasons and can have several benefits including among others:
- Improved dissolving: Tablets and capsules can dissolve in the gut better, allowing your body to absorb it more easily.
- Stability: Keeping the drug mixture stable.
- Improved tolerability: Enhancing things like taste profile so that drugs are easier to swallow.
Importantly, several excipients can help the drug to get across the gut wall, which is especially important in making tablets of peptide drugs (chains of amino acid molecules) that otherwise have to be injected due to their large size. However, the use of these excipients is highly controversial as there is a view that some of them may alter helpful gut bacteria and remove the protective mucus that lines the gut wall, factors that can lead to autoimmune diseases such as inflammatory bowel disease.
For the BUCCAL-PEP project, we need to pay close attention to this as we consider using such agents in our patches designed for cheek attachment with the aim of getting peptides into the blood by that administration route.
Read the full paper here.